
What Medical Device Regulation Might Mean for 3D Printed Patient-Specific Implants - 3DPrint.com | The Voice of 3D Printing / Additive Manufacturing

Medical device regulation encompasses all laws and rules with regards... | Download Scientific Diagram

Medical device regulation in Europe – what is changing and how can I become more involved? - EuroIntervention

HealthTech - Shifting Landscapes Series (Part 2): Software as a Medical Device, Hannah Gardiner, Eliza Saunders
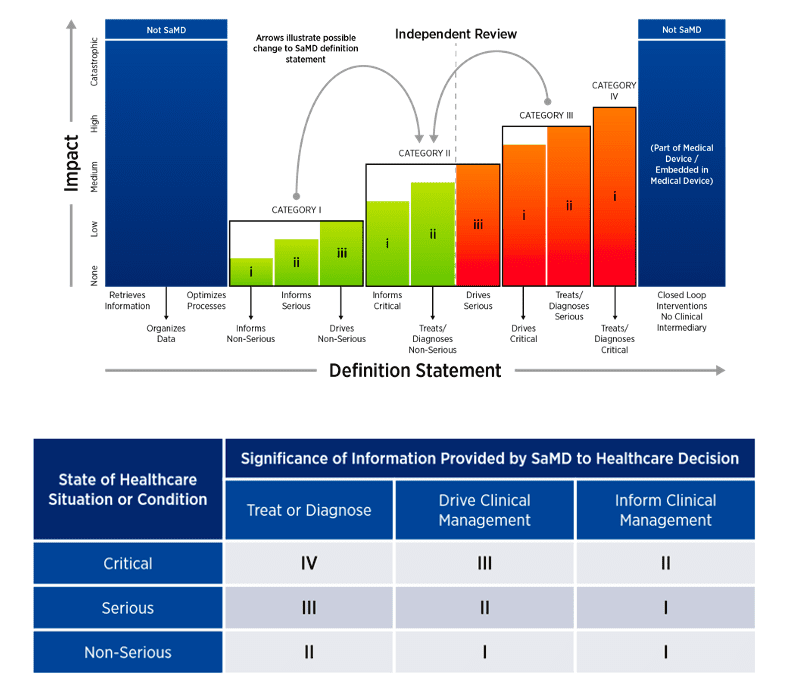
Software as a medical device: Here's how the regulatory landscape is changing - Medical Design and Outsourcing





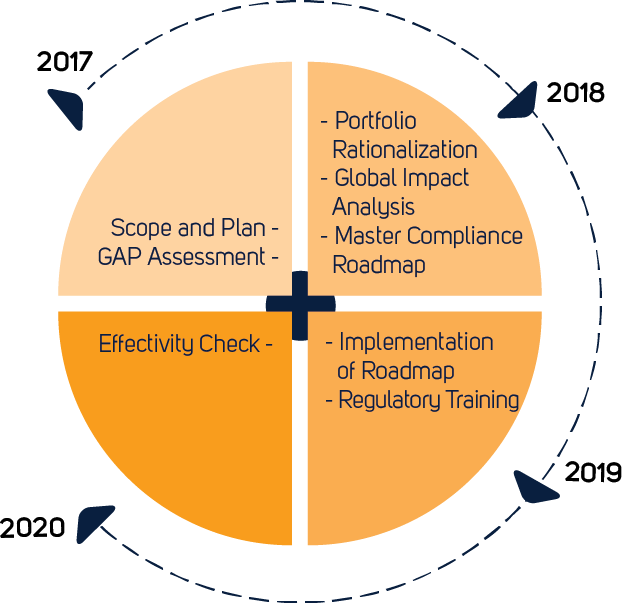




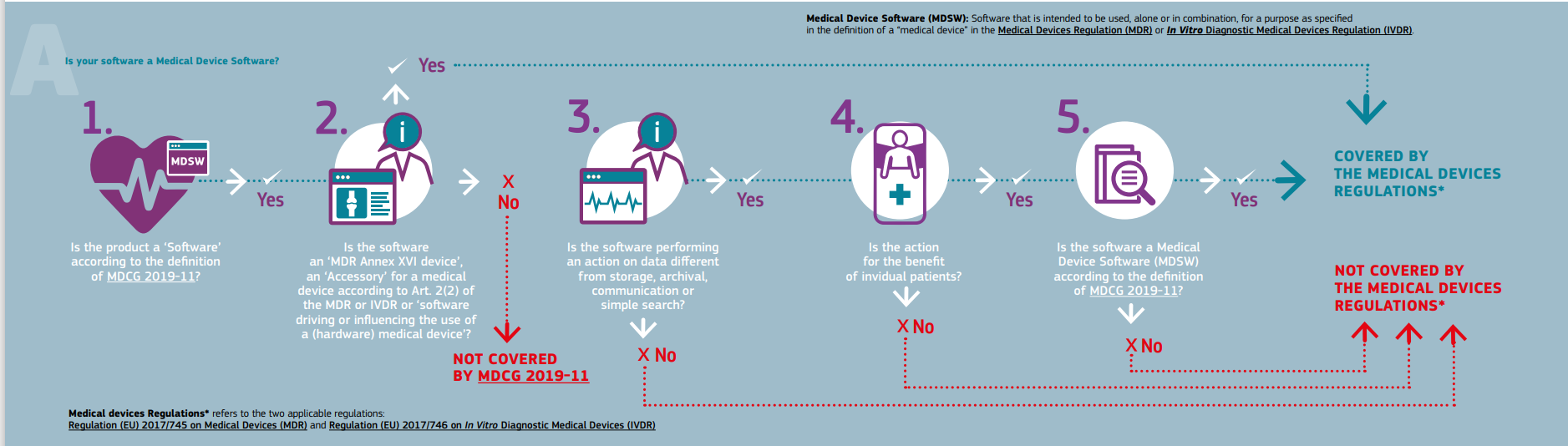

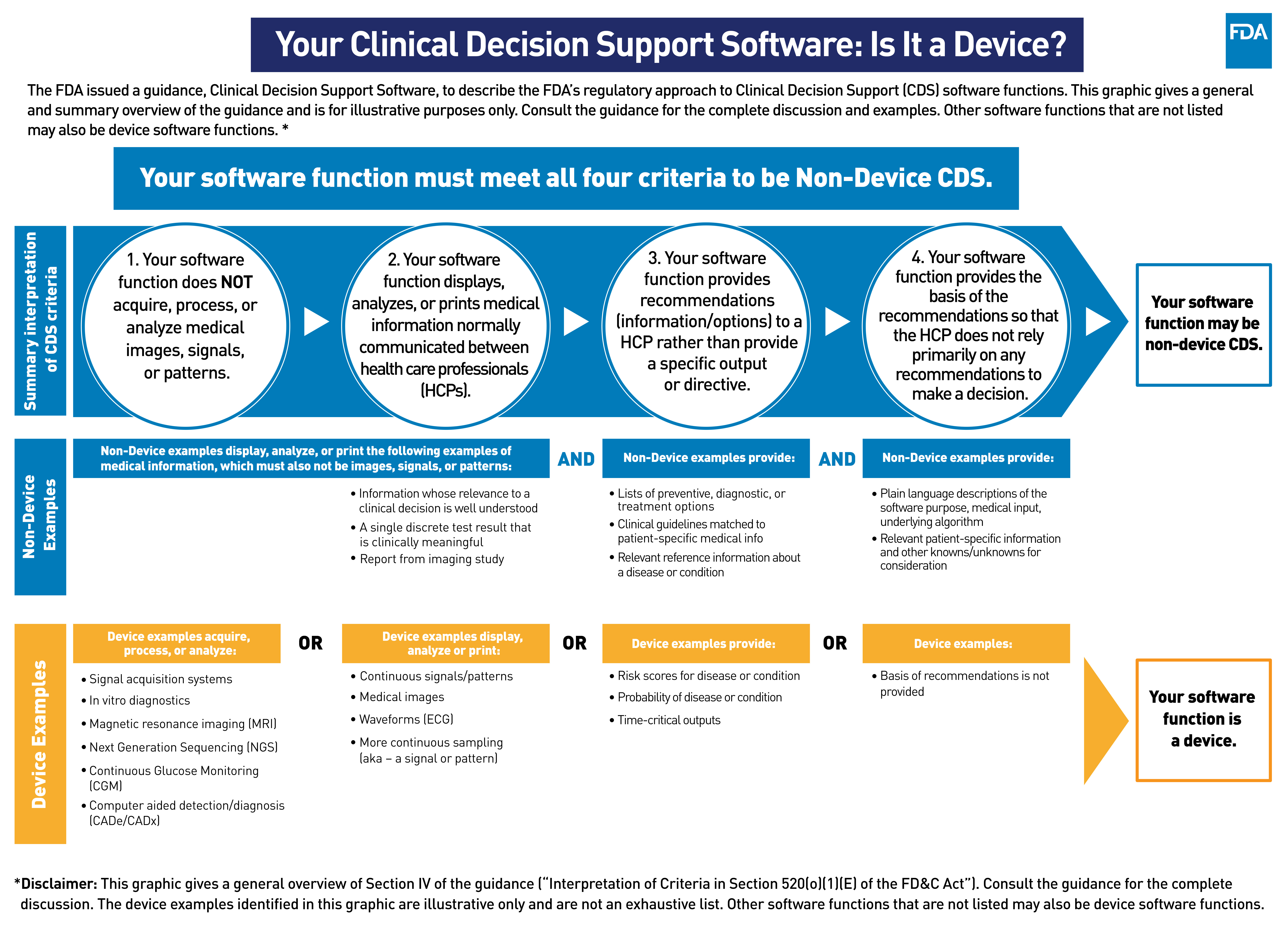



![PDF] Medical Device Regulations: A Current Perspective | Semantic Scholar PDF] Medical Device Regulations: A Current Perspective | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/a80d51106c49bf628b25b6f3c5d565f087c19fea/4-Table2-1.png)