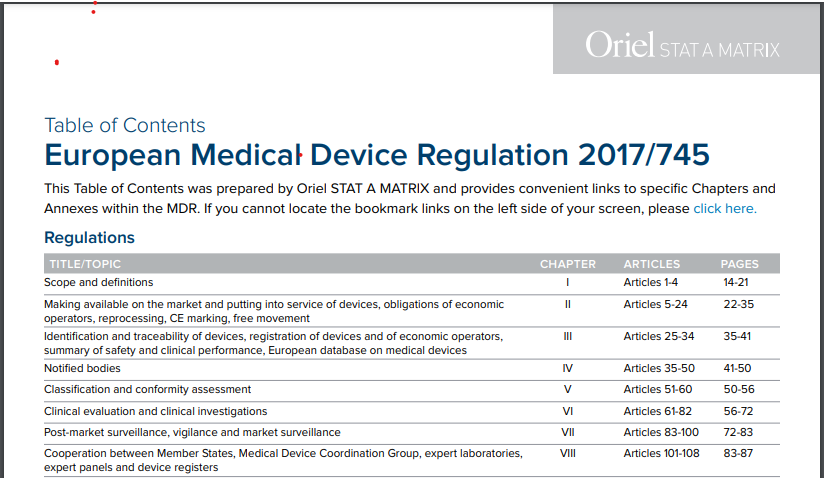
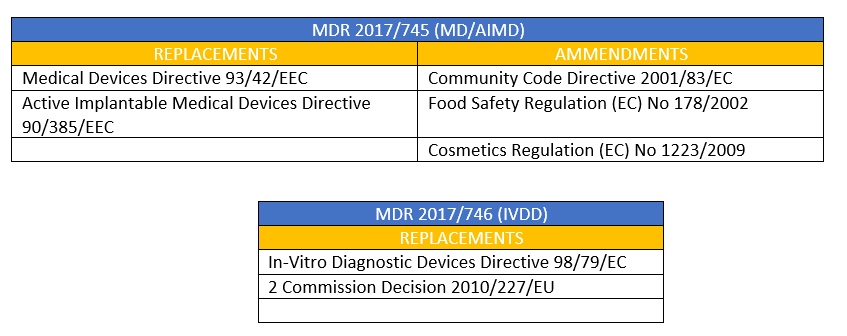
Preguntas más frecuentes sobre la nueva Regulación de Dispositivos Médicos de la UE - B Medical Systems (ES)
REGULATION (EU) 2017/ 745 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL - of 5 April 2017 - on medical devic

The EU's Medical Device Regulation (EU) 2017/745 – Are You Ready for Huge Sweeping Changes? - In Compliance Magazine

![EU MDR 2017/745 Transition timeline [Medical Device Regulation] EU MDR 2017/745 Transition timeline [Medical Device Regulation]](https://easymedicaldevice.com/wp-content/uploads/2018/10/MDR-Transition-timelineV1Slice_01.png)





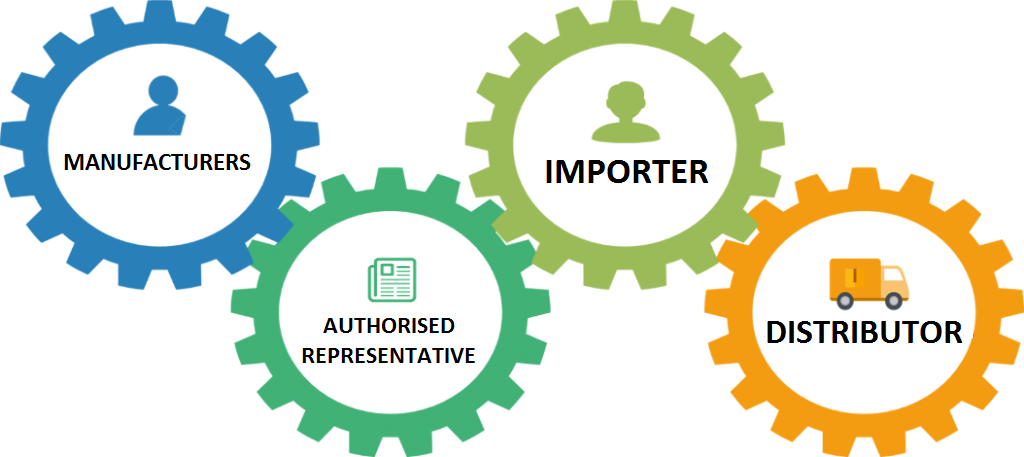

![EU MDR 2017/745 Transition timeline [Medical Device Regulation] EU MDR 2017/745 Transition timeline [Medical Device Regulation]](https://easymedicaldevice.com/wp-content/uploads/2018/10/MDRTransition_update.jpg)