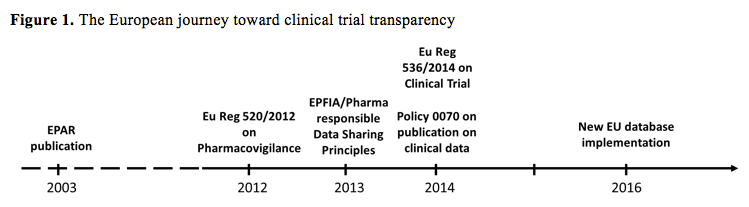
The implementation of the Clinical Trial Regulation (Regulation (EU) No 536/ 2014): where do we stand?
Guide to Clinical Trials Regulation (EU) No. 536/2014 Pilot Project - Ireland Voluntary Pilot Project for the Processing of App

GCP and Quality in “Regulation (EU) 536/2014 on clinical trials on medicinal products for human use and repealing Directive 2001/20/EU” - ScienceDirect
Presentation - Key features and objectives of Regulation EU No 536 /014 - What is new, what has changed? (Laura Pioppo)

Entra en vigor el Reglamento 536/2014 sobre ensayos clínicos de medicamentos y la Comisión publica un FAQ @EU_Health

Europe - MDCG 2022-10 Q&A on the interface between Regulation (EU) 536/2014 on clinical trials for medicinal products for human use (CTR) and Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR) - RIS.WORLD