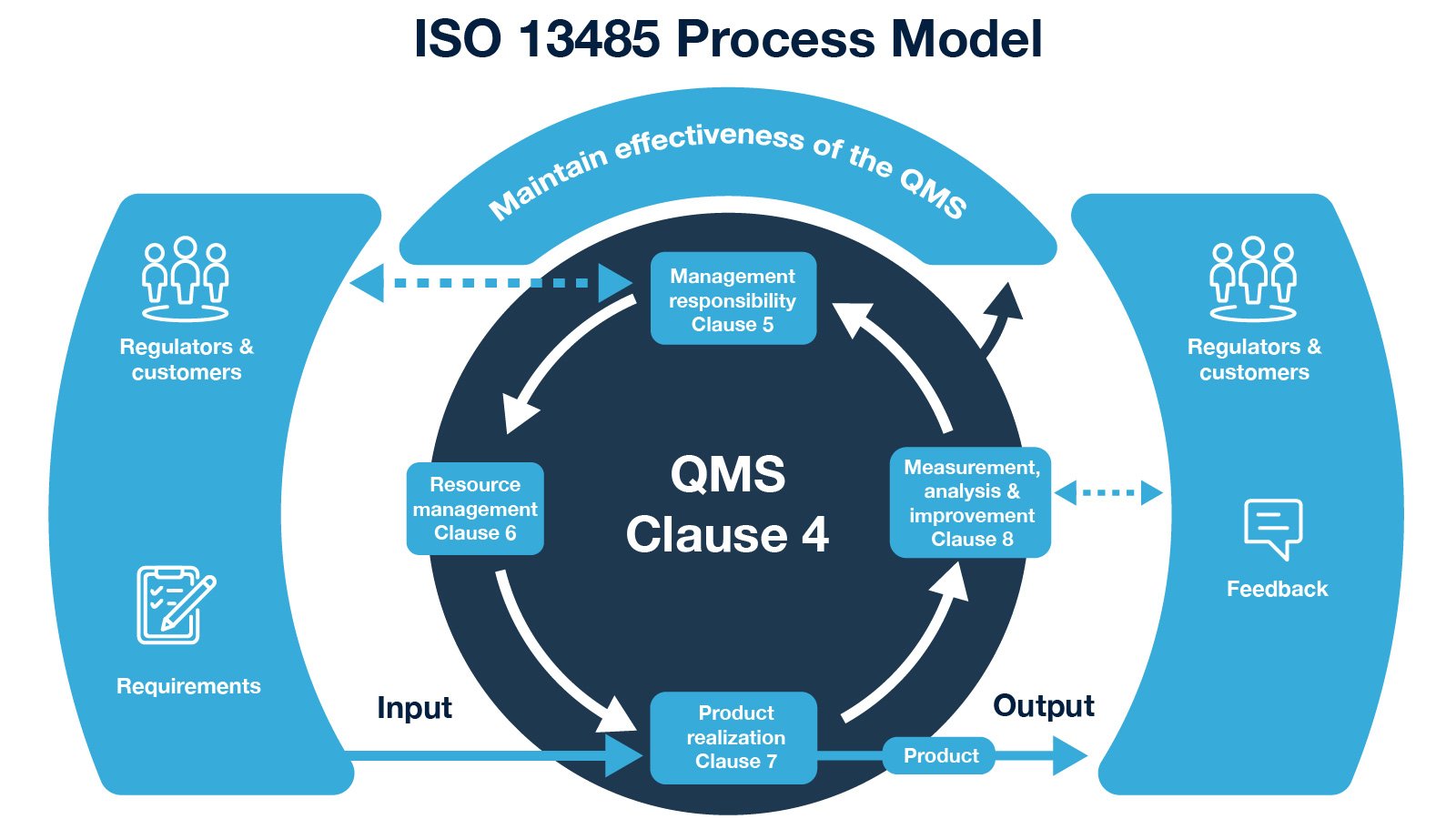
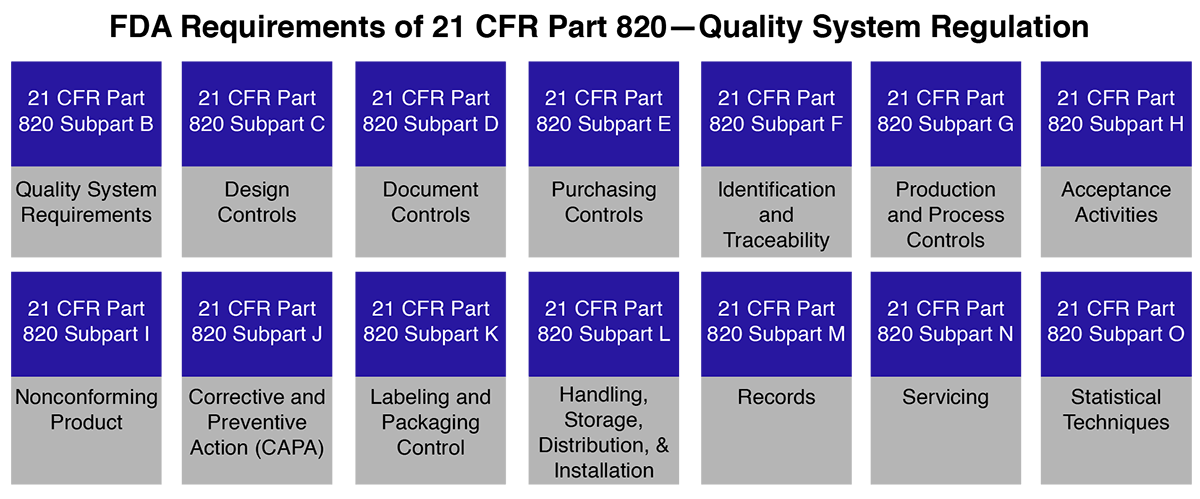
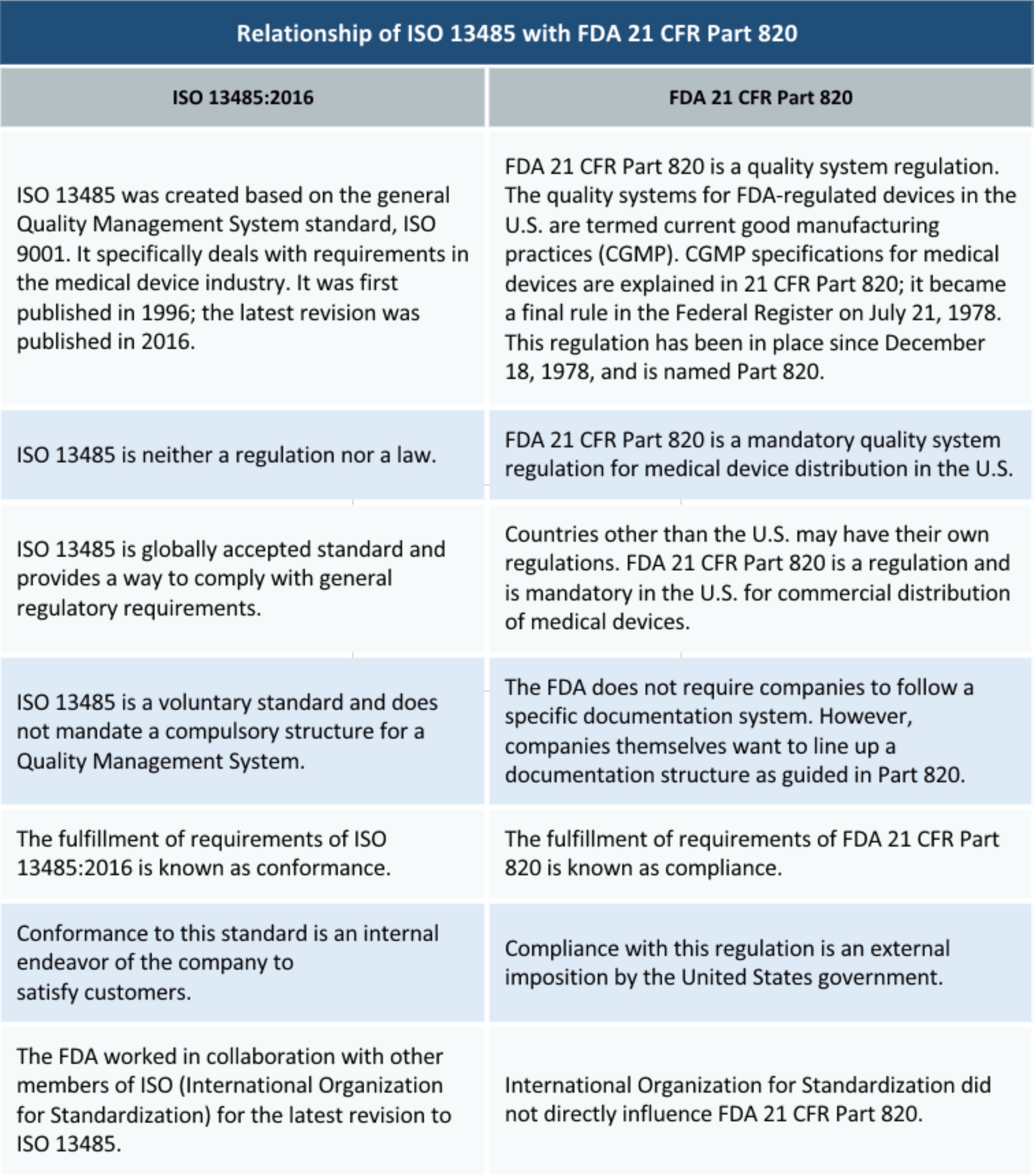
21 CFR Part 820 - Quality System Regulation | 21 CFR 820.30 Medical Device Design Control Guidelines - YouTube

FDA Quality System Regulation: 21 CFR Part 820 Deutsch/Englisch (Beuth Pocket) (German Edition) eBook : Briest, Arne, DIN e.V.: Amazon.es: Tienda Kindle

Us Fda 21 Cfr Part 820 Quality System Regulation Consultant Service in Pimpri Colony, Pimpri Chinchwad, Operon Strategist | ID: 17525804888

FDA Quality System Regulation for Medical Devices (21 CFR Part 820): A Practitioner's Guide to Management Controls (English Edition) eBook : Daugherty, D: Amazon.es: Tienda Kindle