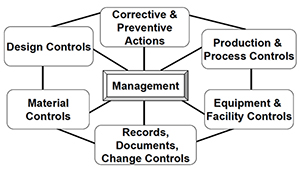
FDA Quality System Regulation for Medical Devices (21 CFR Part 820): A Practitioner's Guide to Management Controls (English Edition) eBook : Daugherty, D: Amazon.es: Tienda Kindle

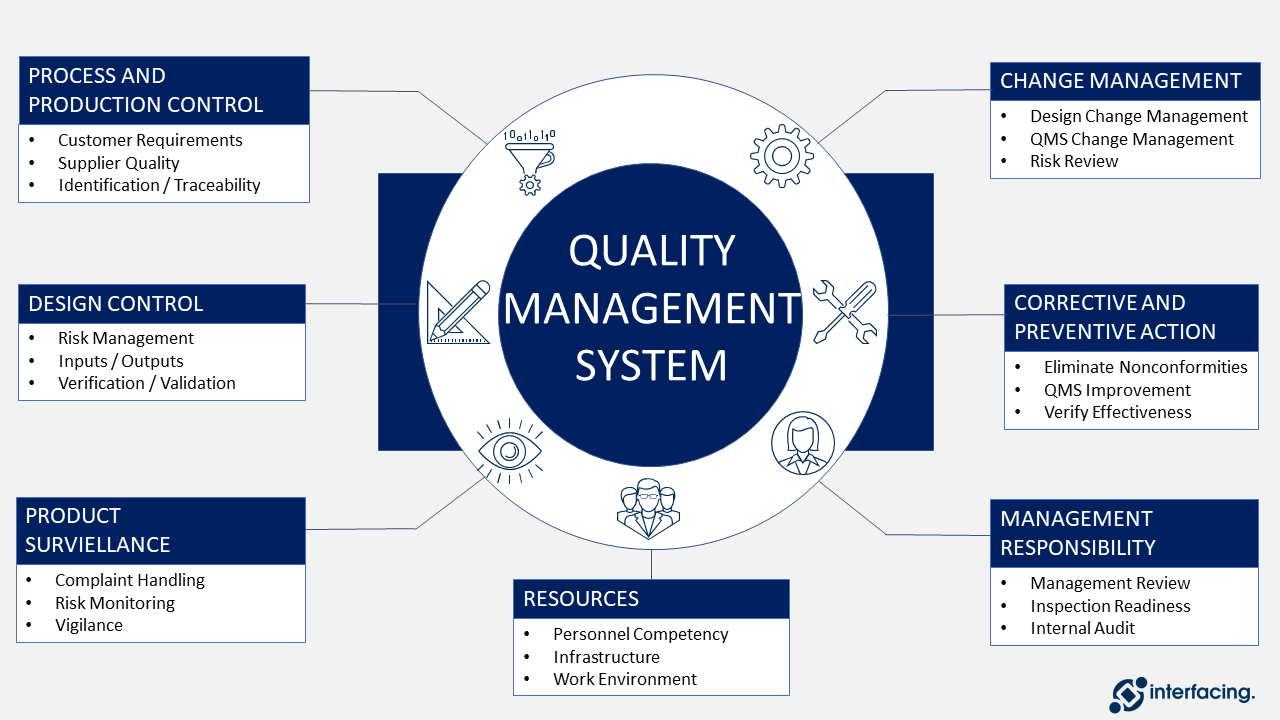
FDA GMP Quality System Regulation: Handling, Storage, Distribution and Installation. : PresentationEZE
%20Explained%20What%20FDA%20QSR%20&%20ISO%2013485%C2%A0Harmonization%20Means%20for%20Medical%20Device%20Companies.png)
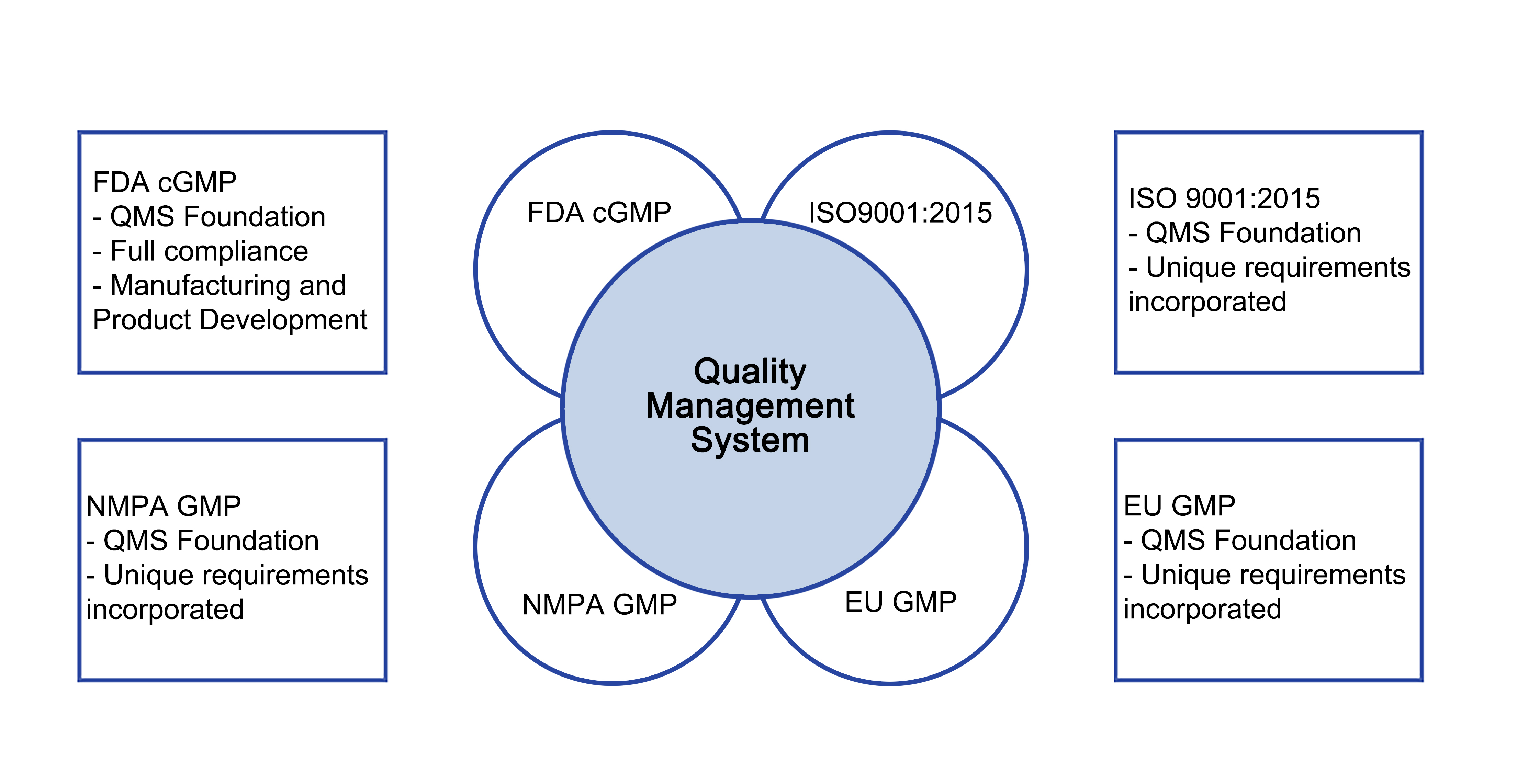
QMSR (Quality Management System Regulation) Explained: What FDA QSR & ISO 13485 Harmonization Means for Medical Device Companies

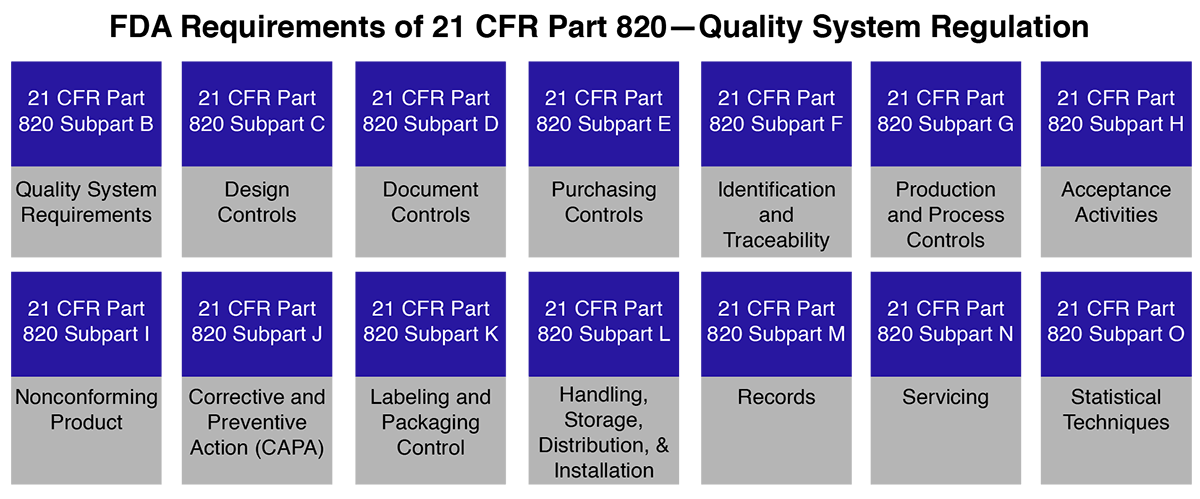
Calaméo - FDA guidance “The Quality Systems Approach To the Pharmaceutical Good Manufacturing Practices"