COMMISSION REGULATION (EU) 2017/ 1410 - of 2 August 2017 - amending Annexes II and III to Regulation (EC) No 1
EU Amended the ANNEX II, III and V of Cosmetics Regulation on CMR Substances - Industry News - C&K Testing

EU Amends Annex II and III of Regulation (EC)1223/2009 on Cosmetic Products - Industry News - C&K Testing
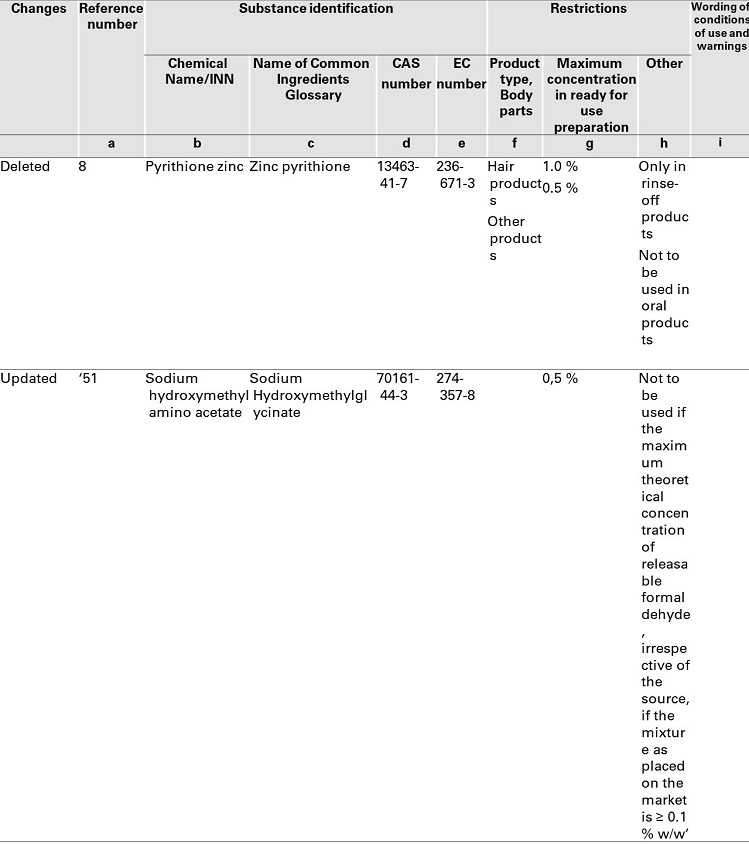
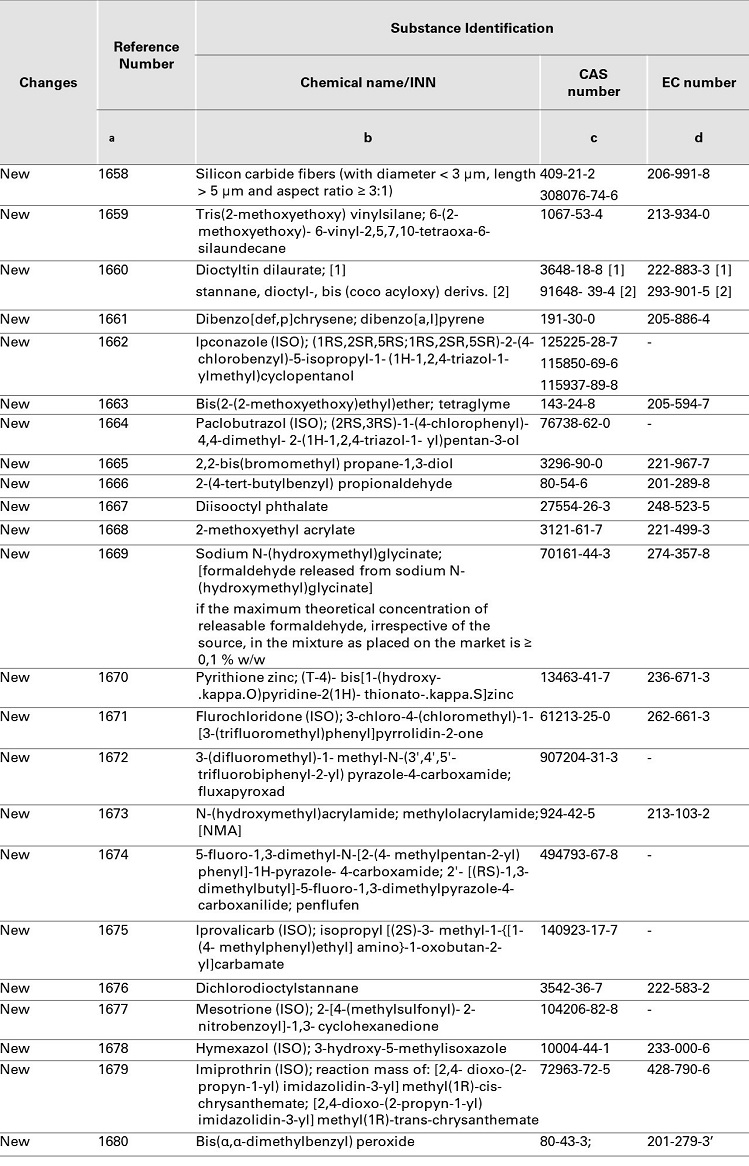
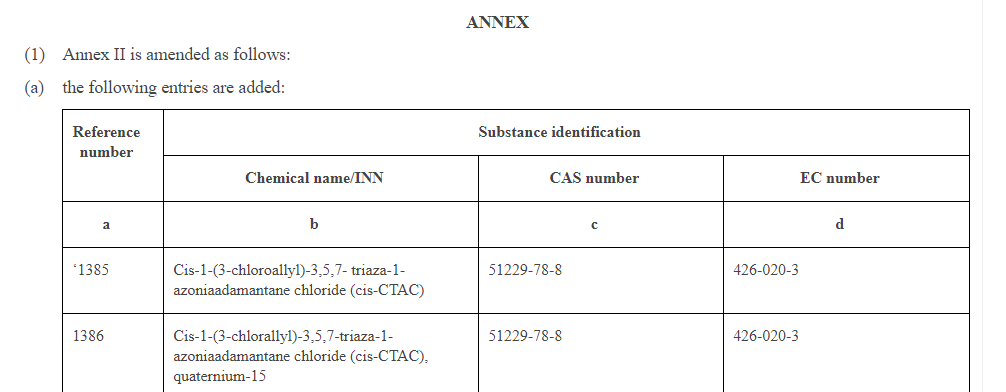
COMMISSION REGULATION (EU) 2019/ 831 - of 22 May 2019 - amending Annexes II, III and V to Regulation (EC) No 1

Regulation 2020/1683: New updates of Annexes II and III to the Cosmetic Regulations for hair dyes - European Commission

Finnish Safety and Chemicals Agency (Tukes) Restrictions for Certain Substances, CMR Substances and Nanomaterials Jarkko Loikkanen, Senior Officer TAIEX. - ppt download
LEGISLATION OF COSMETICS IN THE EU Key Features of Regulation 1223/2009/EU DR.G.R Cosmetics Europe, Brussels, B

EU Amends Annex II and Annex VI to Regulation (EC) No 1223/2009 on Cosmetic Products - Industry News - C&K Testing